
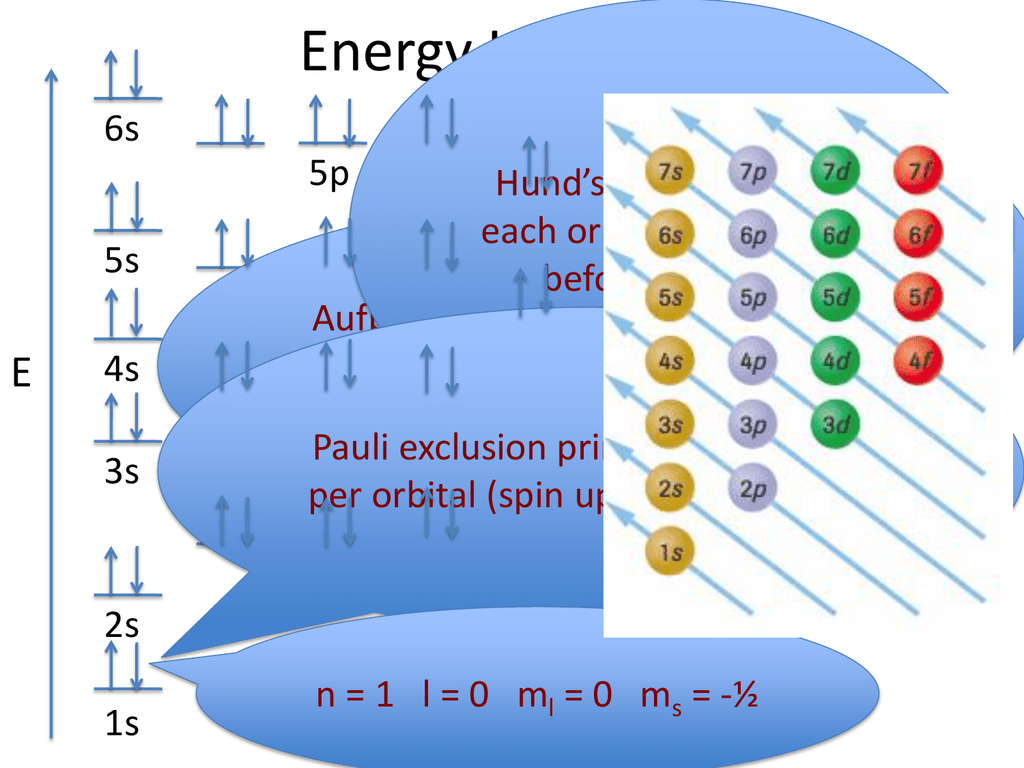
For example, 1s fills first, then 2s, then 2p.
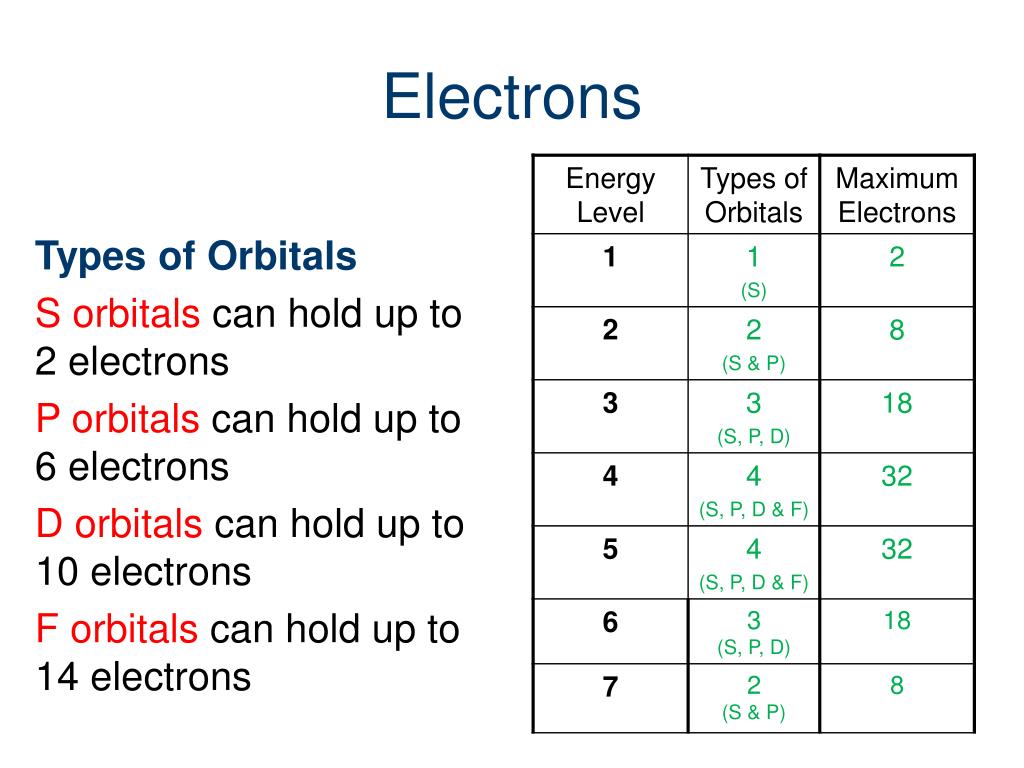
Aufbau principle: shells / subshells of lower energy gets filled first (This is the most obvious rule. Conventional notation for electronic structure. For example, the d subshells have 10 columns showing that d orbitals can hold 10 electrons total. The number of columns for each subshell indicate the maximum number of electrons that subshell can hold. For example, the fifth column of the d subshells contain elements that have 5 electrons in that subshell. For a given subshell, the columns represent how many electrons are in that subshell. The pattern we get from looking at the periodic table is exactly in the order of increasing energy. Starting from the first row, going across, both hydrogen and helium is 1s. The best way to memorize the above is by interpreting the periodic table:. Electrons are filled by occupying the lowest energy subshells first. Common names and geometric shapes for orbitals s, p, d. s is the spin quantum number: s is either +1/2 or -1/2. m is the magnetic quantum number: m are integers that range from -l to l, including zero. a low shell with a high subshell may be higher in energy than a higher shell with a low subshell. for a given shell, higher subshells have higher energy. A generalized formula for the above pattern: for any subshell, 4l+2 electrons can be held. each orbital holds a maximum of 2 electrons. l is the angular momentum quantum number: l are integers that range from 0 to n-1. Quantum numbers l, m, s, and number of quantum states (electrons) per orbital. The emission spectrum shifts to a slightly longer wavelength. 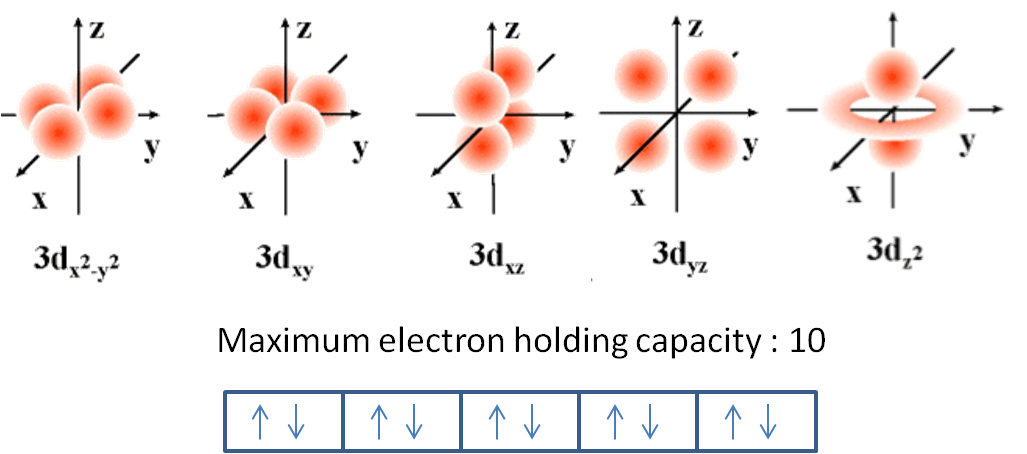
The absorption spectrum corresponds to the emission spectrum in pattern. The emission spectrum looks like colored lines on a black background. The emission spectrum shows what wavelengths of light are emitted. The absorption spectrum looks like black lines on a rainbow background. The absorption spectrum shows what wavelengths of light are absorbed. Excited state electrons come back down to the ground state via release of energy. Excited states are higher in energy than ground states. When they absorb energy, they get promoted to excited states. Electrons are normally in their ground state. Thus, there are 2n^2 electrons per shell. There are n squared orbitals per shell. Higher n shells are higher in energy (if subshells are the same). The principle quantum number, n, defines what shell the electron is in. In quantum mechanics, hydrogen electron exists in a spherical probability cloud around the nucleus. In the Bohr model, the hydrogen electron orbits the nucleus. Orbital structure of hydrogen atom, principal quantum number n, number of electrons per orbital.










